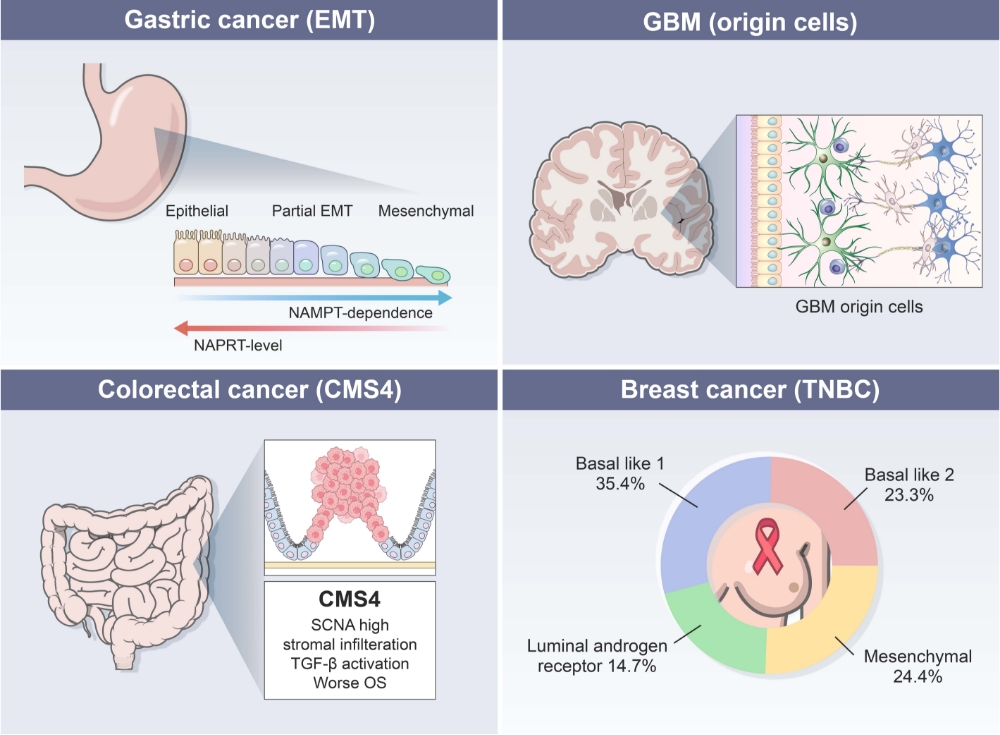
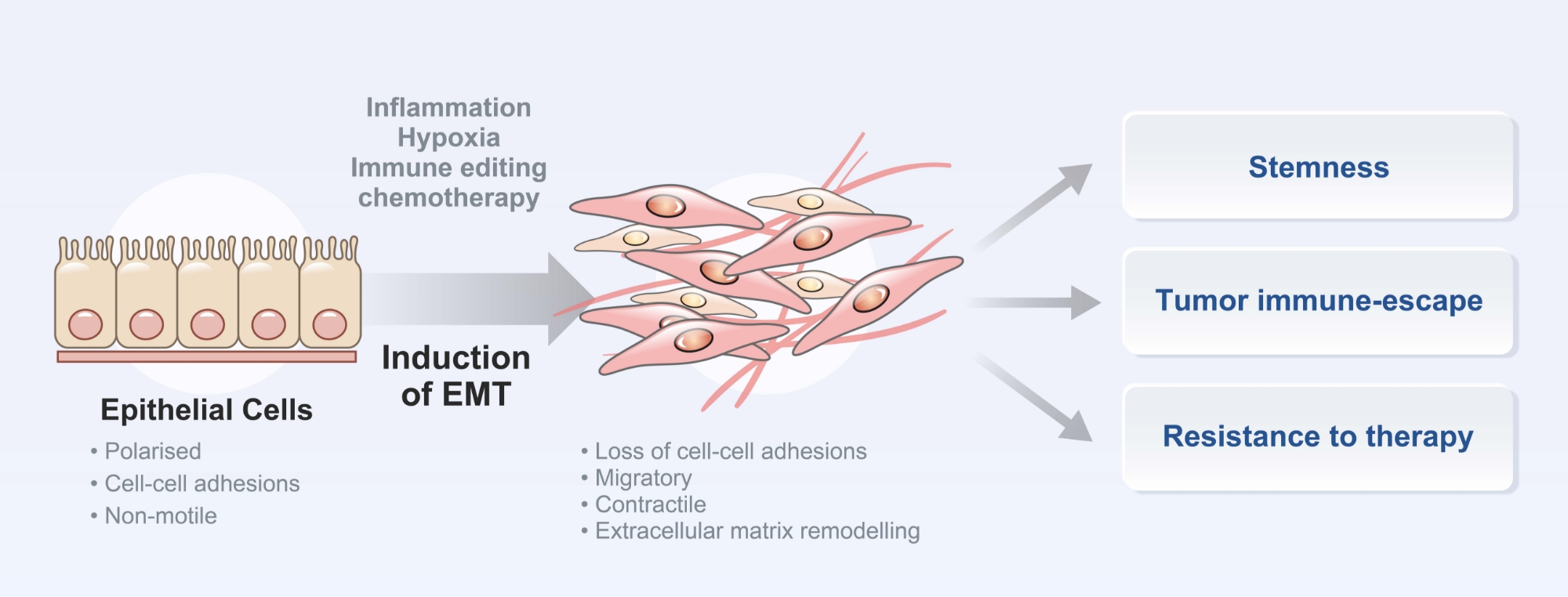
Treatment-refractory tumor models
Treatment-refractory solid tumors pose a significant challenge, as they have a poor prognosis and limited effective treatment options. Most well-known molecular mechanisms underlying treatment-resistance is epithelial-mesenchymal transition (EMT). To address this challenge, CMTX focuses on developing small molecule therapeutics specifically designed to target treatment-refractory cancer subtypes associated with EMT, such as gastric cancer with EMT subtype, colorectal cancer with CMS4 subtype, mesenchymal subtype of triple negative breast cancer, and glioblastoma tumor initiating cells etc.